Abstract
Introduction: Telomere Biology Disorders (TBD) are due to germline variants in telomere maintenance and repair genes. Clinical manifestations include bone marrow failure (BMF), liver and lung fibrosis, and risk of cancer, especially myeloid neoplasia. We characterized clinical phenotype, pathology, and clonal landscape of patients with TBD and myelodysplastic syndrome (MDS) or acute leukemia (AL). We assessed if somatic variants in myeloid cancer genes are associated with MDS/AL or overall survival (OS).
Methods: Patients (n=109) from the National Institutes of Health and University of São Paulo (NIH/USP) (n=91) or MD Anderson (MDA) (n=18) met TBD clinical criteria or had a pathogenic/likely pathogenic germline variant in a telomere maintenance/repair gene. All patients gave informed consent. Clinical characteristics and details of hematologic malignancy were collected. MDS was defined using WHO 2016 criteria. In the NIH/USP cohort, somatic variants in myeloid cancer genes were assessed in peripheral blood using error-corrected DNA sequencing (ECS; minimum variant allele frequency [VAF] of 0.5%) and in the MDA cohort patients were screened by targeted amplicon-based next-generation sequencing (minimum VAF of 2%). Serial samples were available in 31 (41%) patients.
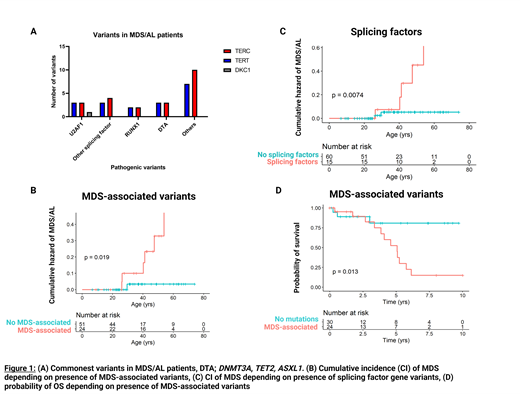
Results: Eighteen out of 109 (17%) patients from both the NIH/USP and MDA cohorts had a diagnosis of MDS/AL, developing in patients with TERC (n=8/31; 25%), TERT (n=8/48; 16%), RTEL1 (n=1/5; 20%), and DKC1 (n=1/2; 50%). Karyotypes were normal diploid (n=6), chromosome 1 abnormality (n=5), trisomy 8 (n=2), deletion 20q (n=1), complex (n=2), monosomy 7 (n=1) and deletion 7q (n=1). Six patients had >5% blasts. Four patients had concurrent liver fibrosis and 9 had pulmonary fibrosis. Somatic mutation data at time of MDS/AL was available for 16/18 patients; splicing factor genes (n=14) were most frequent, seen in 11 (69%) patients, with U2AF1 predominating (n=7; 44% of patients) (Figure 1A). More than 1 mutation was present in 12/16 (75%) of patients. One patient developed AML 6 years after MDS diagnosis . U2AF1 variants were also present in 8 patients without MDS/AL; 5 had no sequential samples and 3 had stable mutations over 2 (n=2) and 4 (n=1) years. Recurrent variants in U2AF1 were present at p.S34 (n=12) and p.Q157 (n=2). Median U2AF1 variant allele fraction (measured in NIH/USP cohort only) was 23% in patients with MDS/AL compared to 7% in those without.
Variant association analysis was confined to NIH/USP cohort due to DNA sequencing assay differences; data were available in 75 patients. Patients were divided into groups defined by specific variants at any time of sampling; "Clonal-hematopoiesis of indeterminate potential / aplastic anemia (CHIP/AA) associated" (DNMT3A, TET2, ASXLI, or BCOR/L1), "MDS-associated" (containing splicing factor genes, RUNX1, SETBP1, ETV1, KRAS, STAG2, GATA2/1, TP53), "PPM1D" (containing PPM1D), and a subgroup, "Splicing factors" (U2AF1, ZRSR2, SF3B1, SRSF2). Patients with MDS-associated variants (Figure 1A; p=0.02), particularly splicing factors (Figure 1B; p=0.007) more likely had or later developed MDS/AL, compared to patients without somatic variants or in other variant groups. MDS/AL was not associated with PPM1D or CHIP/AA-associated variants. OS from time of first detected somatic variant was lower in the MDS-associated variant group (Figure 1C p=0.013) compared to those without variants at time of first assessment; this was not the case for CHIP/AA-associated variants or PPM1D. Patients with somatic variants (median ages 36, 41, and 42 for PPM1D, CHIP/AA-associated, and MDS-associated respectively) were significantly older than patients without variants (median age 24) though ages were similar within variant groups.
Conclusion: Splicing factor gene variants predominate the clonal landscape of TBD-associated hematologic malignancy; U2AF1 p.S34 is recurrent and can occur in the absence of neoplasia. Splicing factor gene variants such as U2AF1 in the peripheral blood may be potential biomarkers for hematologic malignancy in TBD patients. PPM1D, previously associated with therapy-related MDS, is commonly mutated in TBD but does not associate with MDS/AL. OS was decreased in patients with MDS-associated variants but not with PPM1D or CHIP/AA-associated variants. Further study of U2AF1 variants in TBD patients may give insight to the underlying drivers of MDS/AL.
DiNardo: Agios/Servier: Consultancy, Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Forma: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Takeda: Honoraria; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Calado: Novartis Brasil: Honoraria; Instituto Butantan: Consultancy; AA&MDS International Foundation: Research Funding; Alexion Brasil: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; Team Telomere, Inc.: Membership on an entity's Board of Directors or advisory committees. Young: Novartis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal